What is Low-Resolution Electromagnetic Tomography (LORETA)?
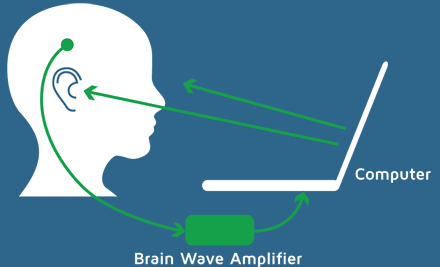
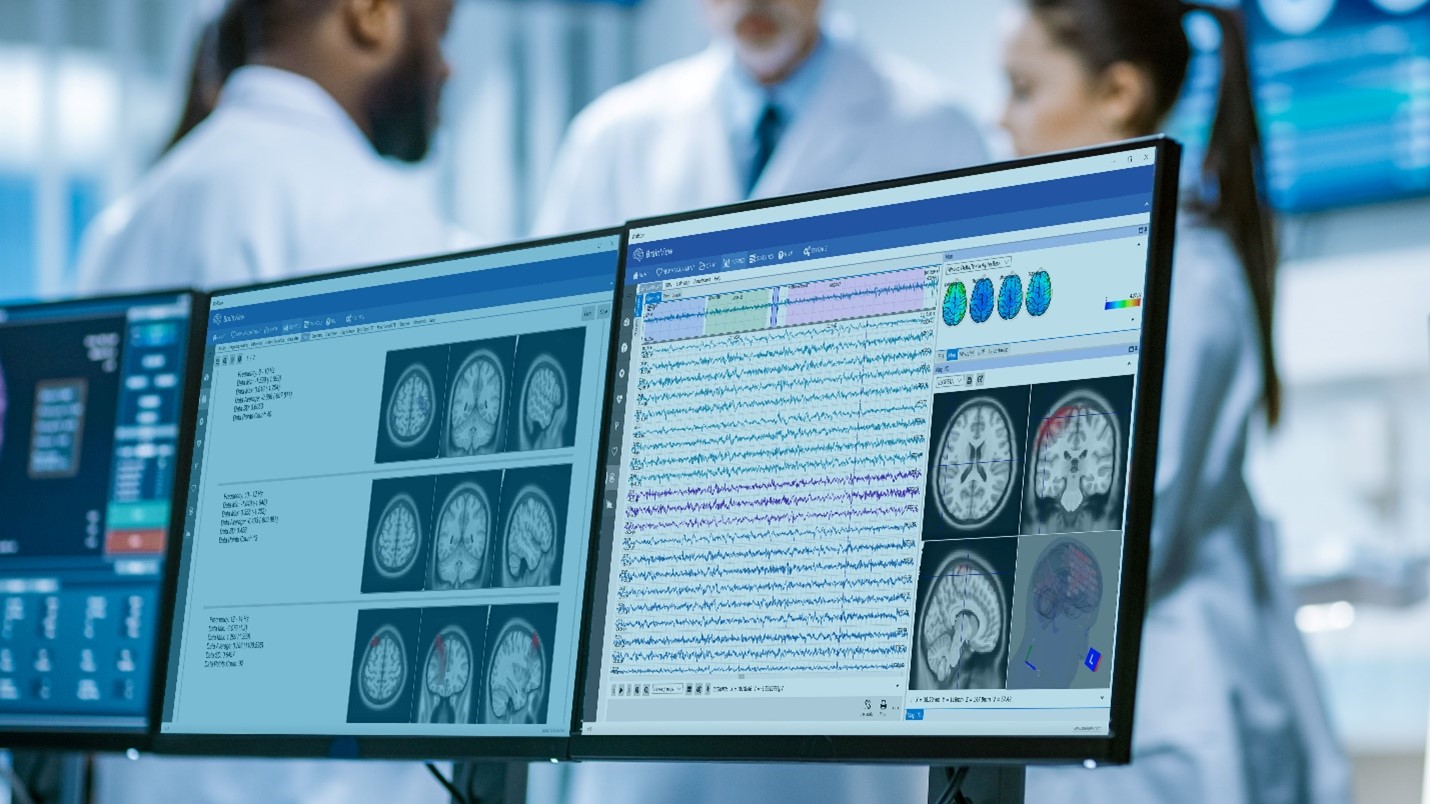
Neuro-imagining techniques aim to represent the structure or functioning of the brain. They can be understood as an X-ray photograph of the brain that, in the case of functional imagining, will show the brain areas activated during a process or cognitive task, and techniques such as EEG or MEG are examples.
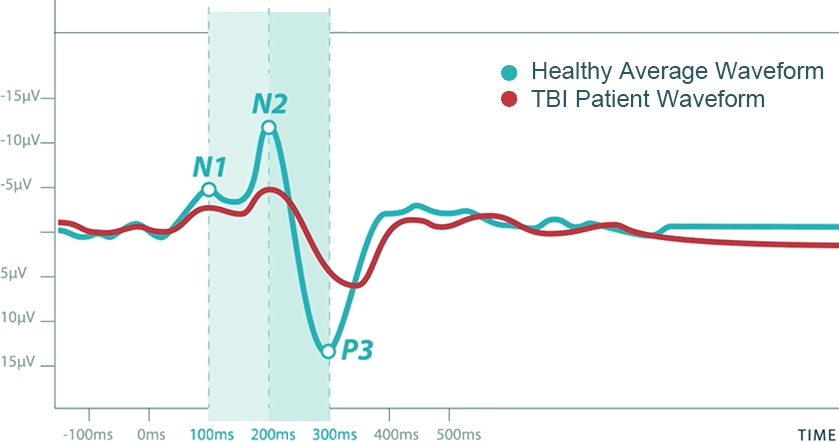
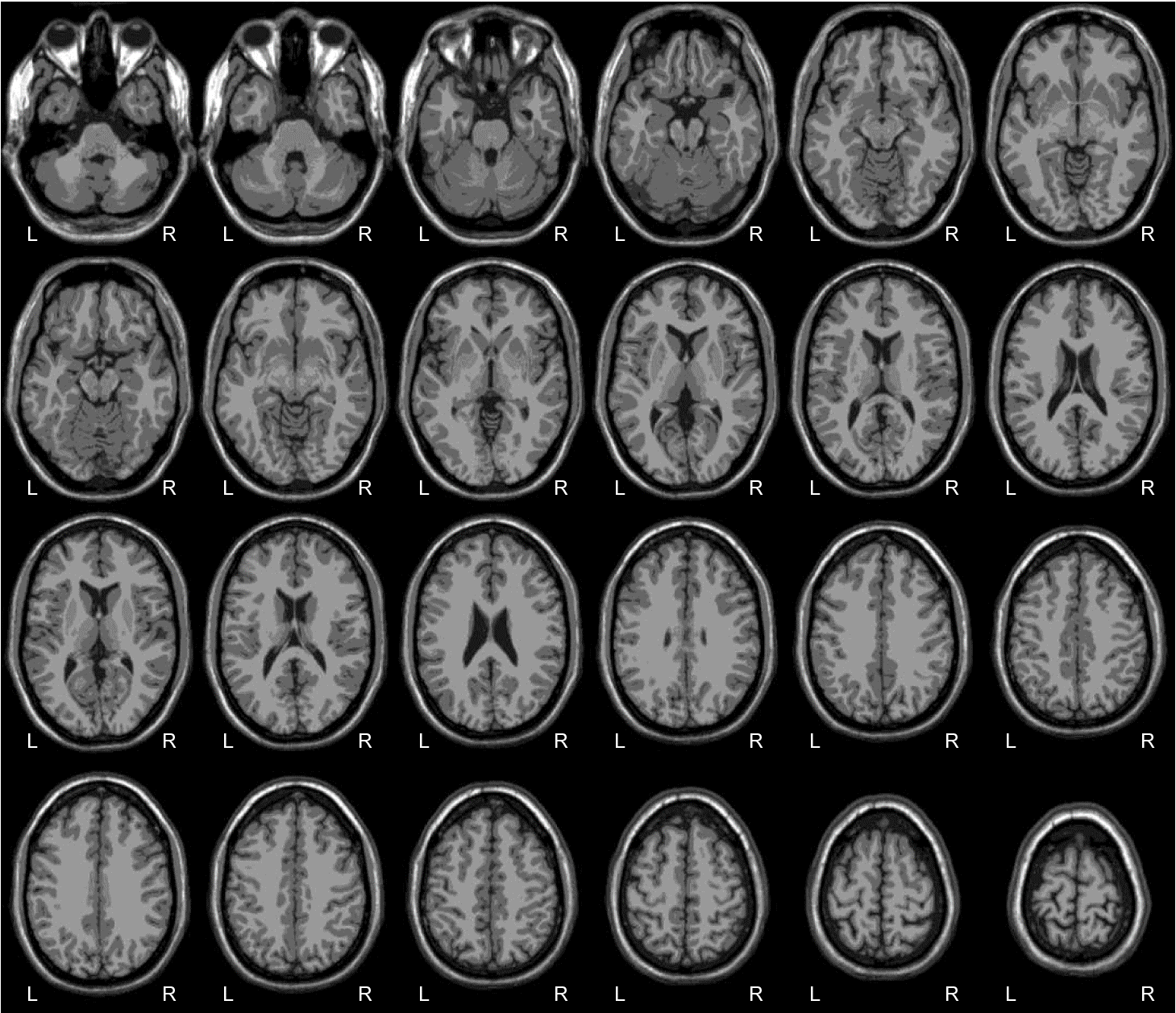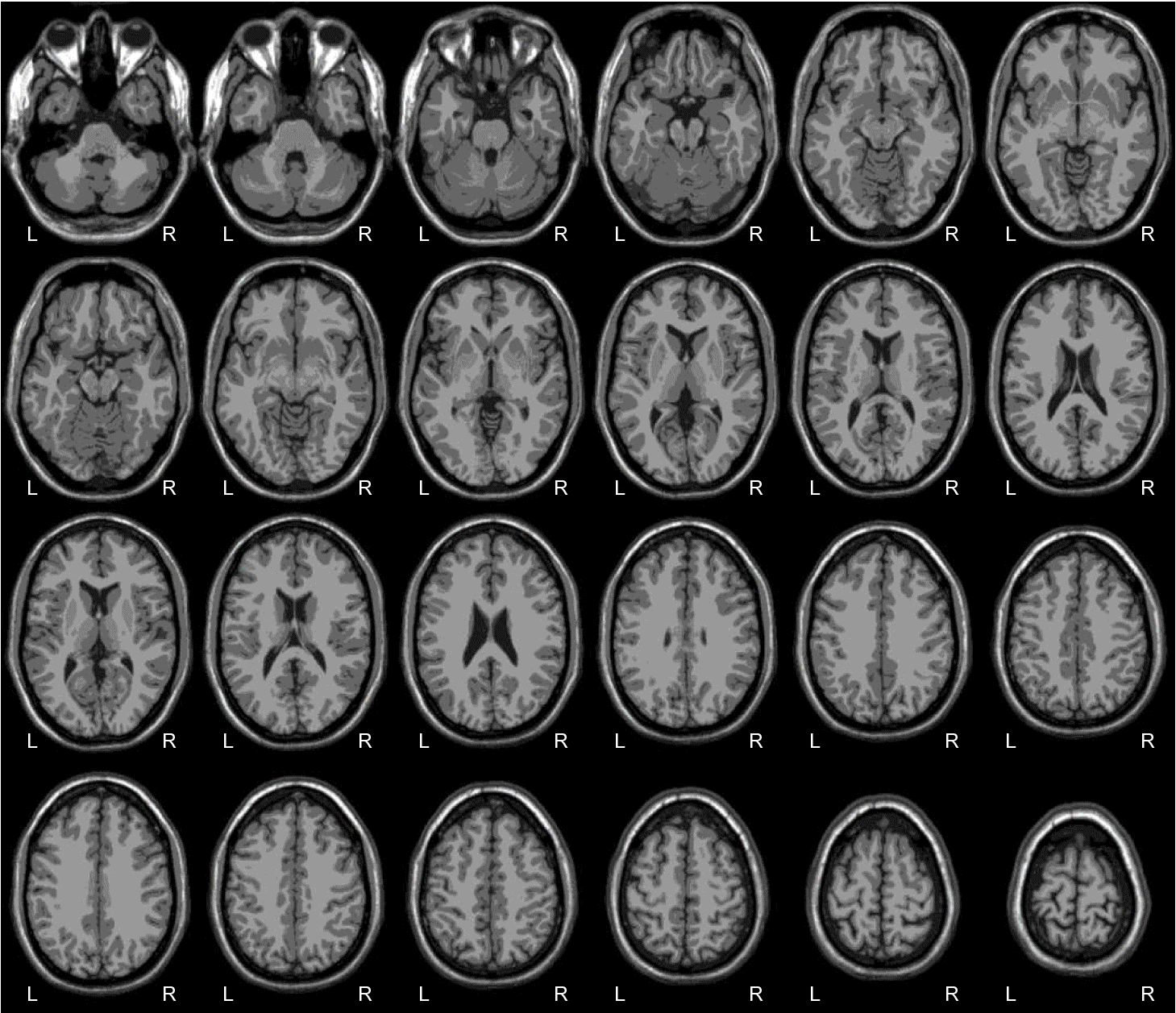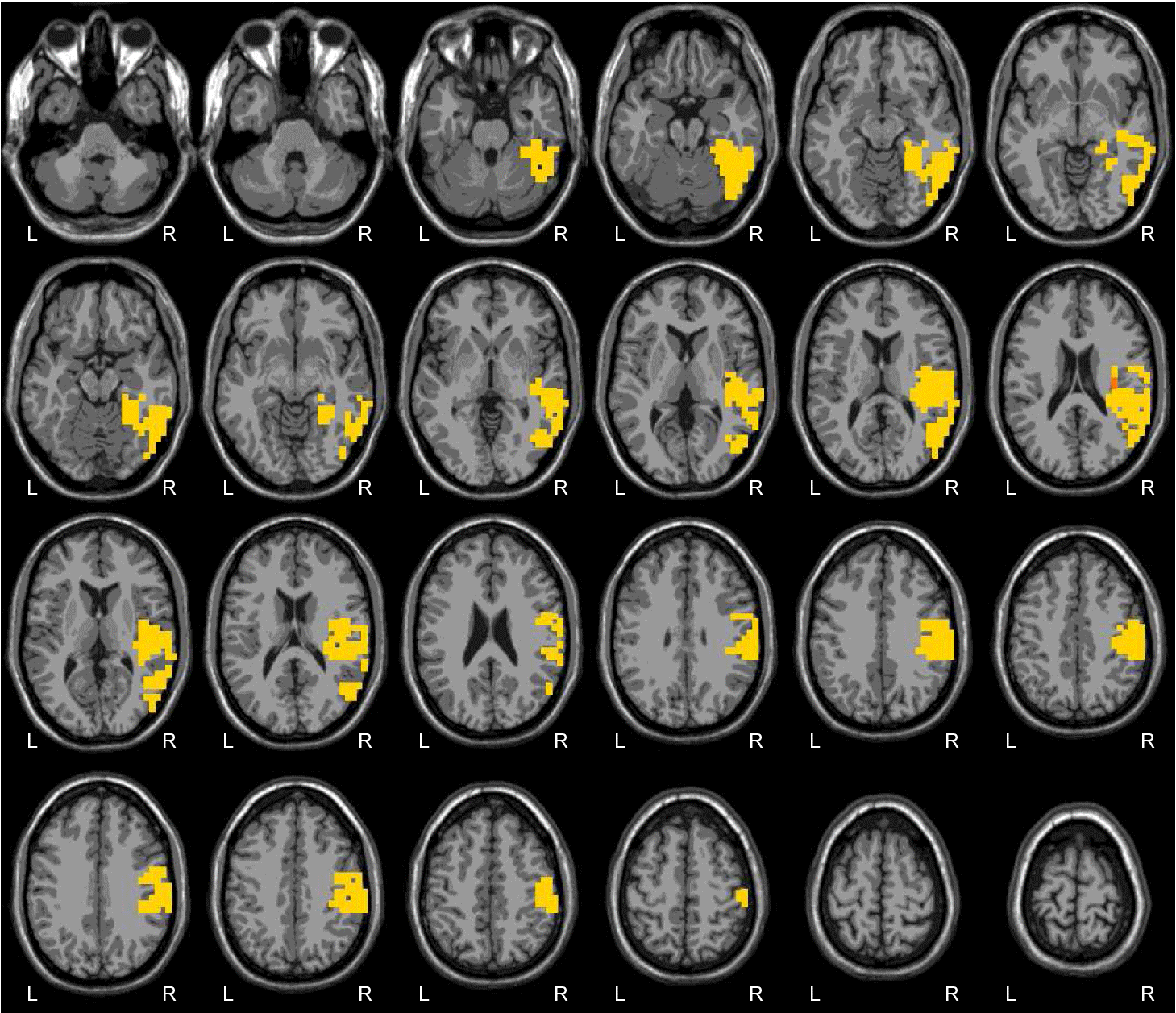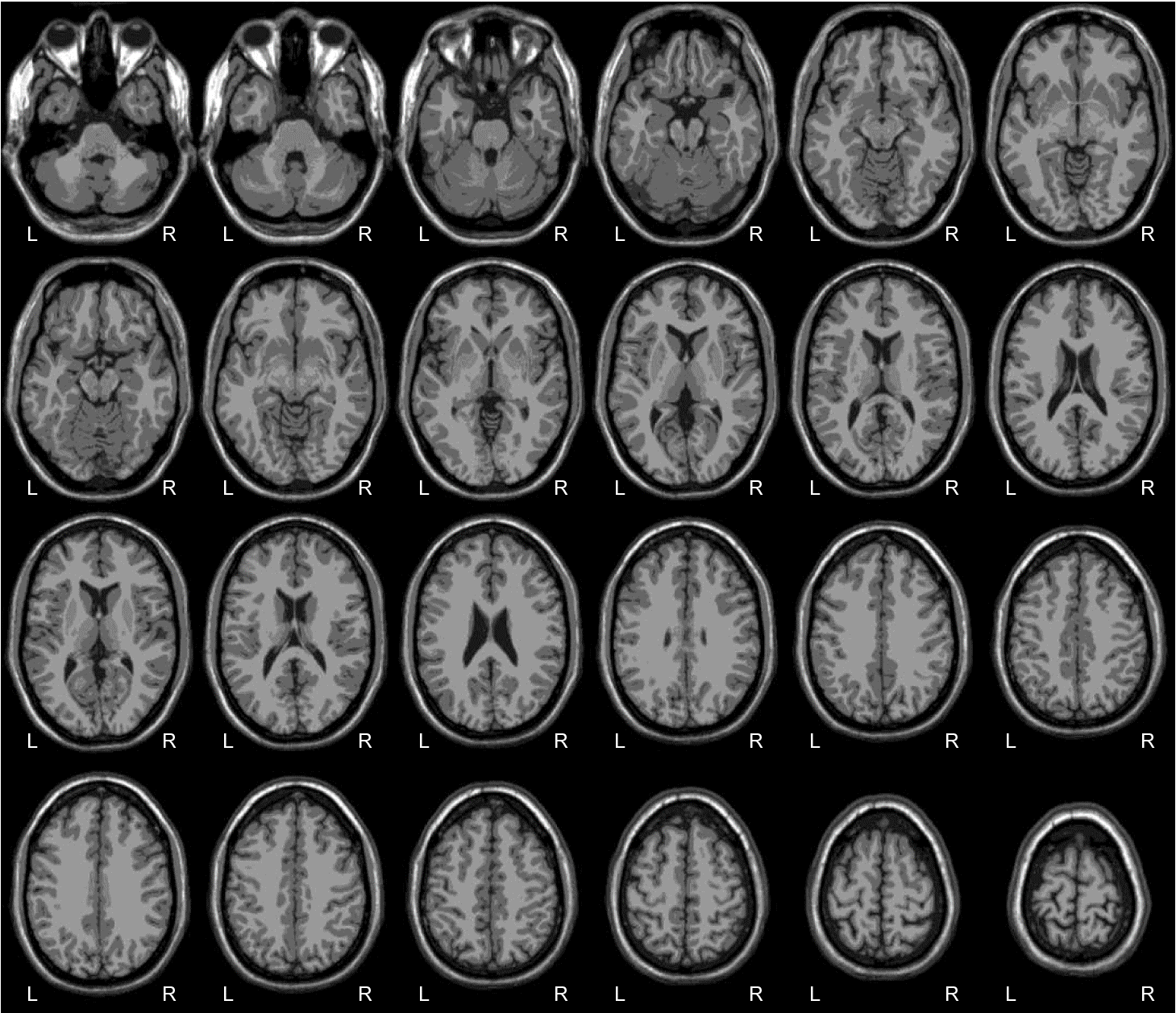
Pascual-Marqui, Michel, and Lehman published in 1994 a new method for localizing the electrical activity in the brain based on scalp potentials from multiple-channel EEG recording. This method solves the inverse problem: convert measurements into information about a physical object or system observed. This revolutionary technique was called LORETA, standing for low-resolution brain electromagnetic tomography, and can be understood as an EEG-based neuro-imagining technique. LORETA computes a three-dimensional distribution of 2400 voxels of 10x10x10mm, generating electric neuronal activity in the grey matter. A significant advantage of this technique is that it is not restricted to a certain number of electrodes or electrode locations. Therefore, it self-adapts to almost every electrode set-up and EEG measuring device.
- sLORETA: standardized low-resolution brain electromagnetic tomography (Pascual-Marqui, 2002). It has no localization bias in the presence of measurement and biological noise.
- eLORETA: fast low-resolution brain electromagnetic tomography (Pacual-Marqui 2005). The first-ever 3D, discrete, distributed, a linear solution to the inverse problem of EEG/MEG with exact localization (zero localization error).
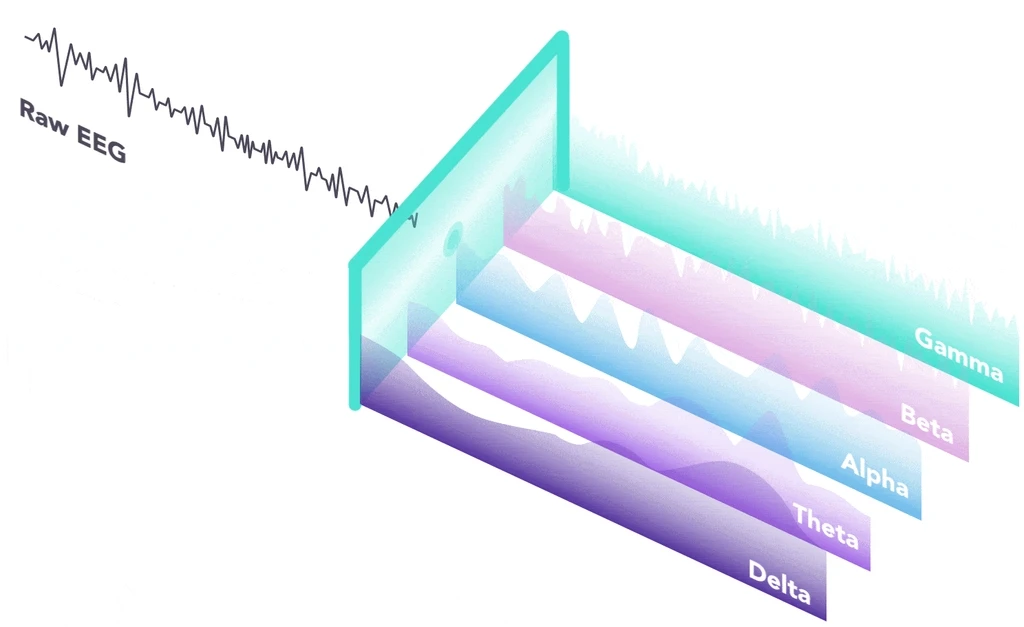
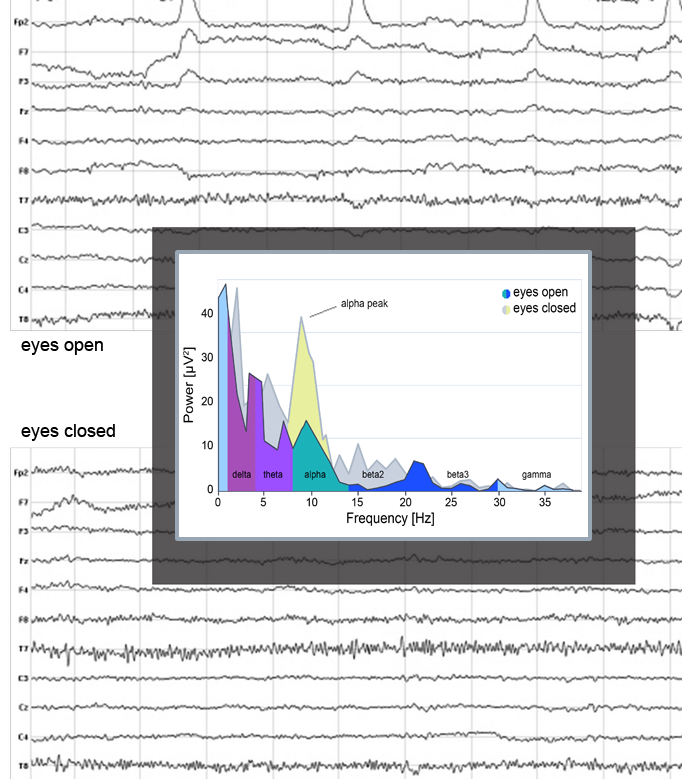
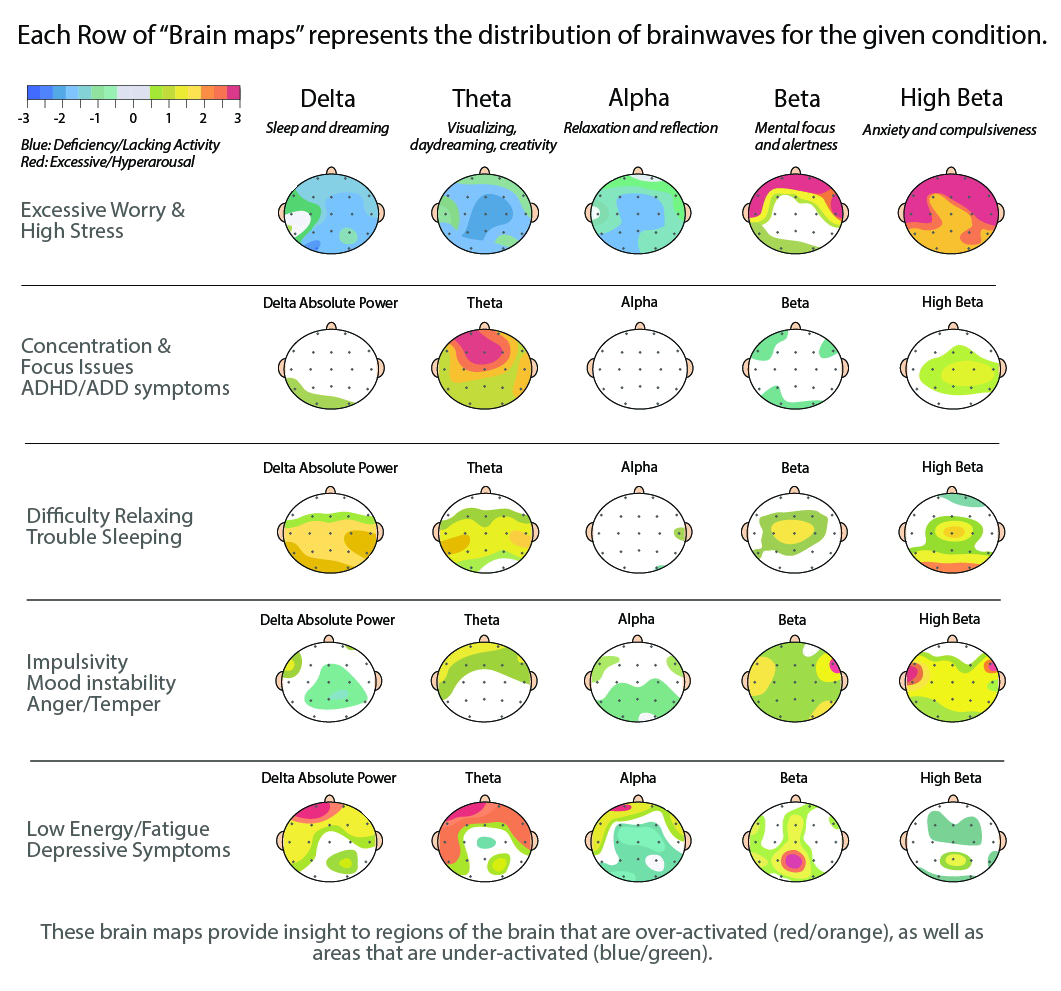
Scalp EEG activity shows oscillations at a variety of frequencies. This rhythmic activity divides into frequency bands, and the most commonly known bands are delta, theta, alpha, beta, and gamma. EEG frequency bands have been noted to have particular biological significance and are associated with different brain functioning states. There are still uncertainties about exactly where various frequencies are generated. However, on the contrary, there is substantial knowledge about the activated areas within the brain that generate specific spectral activity along the scalp. LORETA analysis of particular frequency bands can determine which brain regions are activated during different states or mental tasks, helping determine if the brain operates in an optimal way.